| Product Includes | Product # | Quantity | Mol. Wt | Isotype/Source |
|---|---|---|---|---|
| Cleaved Caspase-9 (Asp330) (E5Z7N) Rabbit mAb | 52873 | 20 µl | 37 kDa | Rabbit IgG |
| Caspase-3 (D3R6Y) Rabbit mAb | 14220 | 20 µl | 35, 19, 17 kDa | Rabbit IgG |
| Caspase-2 (C2) Mouse mAb | 2224 | 20 µl | 12, 14, 48 kDa | Mouse IgG1 |
| Caspase-9 (C9) Mouse mAb | 9508 | 20 µl | 47/37/35 (H). 49/39/37 (M). 51/40/38 (R). kDa | Mouse IgG1 |
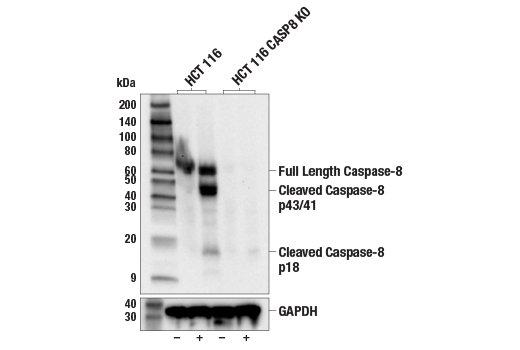
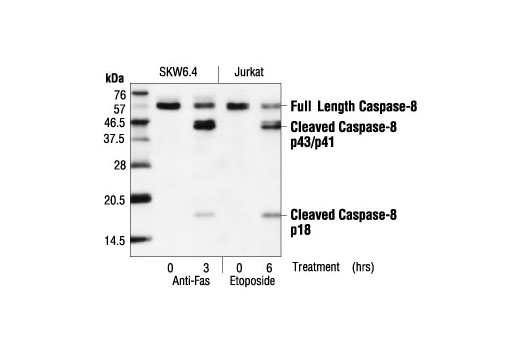
| Caspase-8 (1C12) Mouse mAb | 9746 | 20 µl | 18, 43, 57 kDa | Mouse IgG1 |
| Anti-rabbit IgG, HRP-linked Antibody | 7074 | 100 µl | Goat | |
| Anti-mouse IgG, HRP-linked Antibody | 7076 | 100 µl | Horse |
Please visit cellsignal.com for individual component applications, species cross-reactivity, dilutions, protocols, and additional product information.
Description
The Initiator Caspases Antibody Sampler Kit provides an economical means of evaluating initiator (apical) caspase proteins. The kit contains enough primary antibody to perform two western blots with each primary antibody.
Storage
Background
Apoptosis is a regulated physiological process leading to cell death. Caspases, a family of cysteine acid proteases, are central regulators of apoptosis. Initiator caspases (including 2, 8, 9, 10 and 12) are closely coupled to proapoptotic signals, which include FasL, TNF-α, and DNA damage. Once activated, these caspases cleave and activate downstream effector caspases (including 3, 6 and 7), which in turn cleave cytoskeletal and nuclear proteins such as PARP, α-fodrin, DFF and lamin A; inducing apoptosis (1,2).
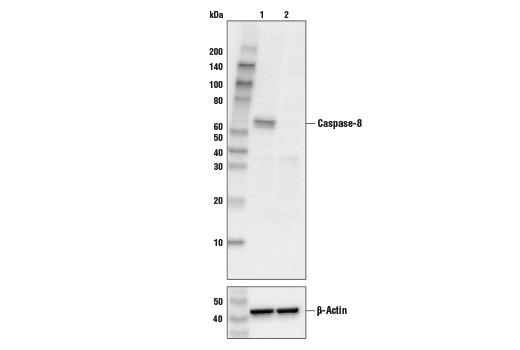
Formation of a death-inducing signaling complex (DISC) around the receptors for death factors, including FasL and TNF-α, is essential for receptor-mediated apoptosis (3). Upon ligand activation, Fas and TNF-R1 associate with death domain (DD) containing adaptor proteins FADD (Fas associated death domain) (4,5) and TRADD (TNF-R1 associated death domain) (6). In addition to a carboxy-terminal DD, FADD contains an amino-terminal death effector domain (DED) that binds to DEDs and activates initiator caspase 8 (FLICE, Mch5, MACH) and caspase 10 (FLICE2, Mch4) (7-12). TRADD does not contain a DED and therefore must associate with FADD in response to TNF-R1 driven apoptosis (13).
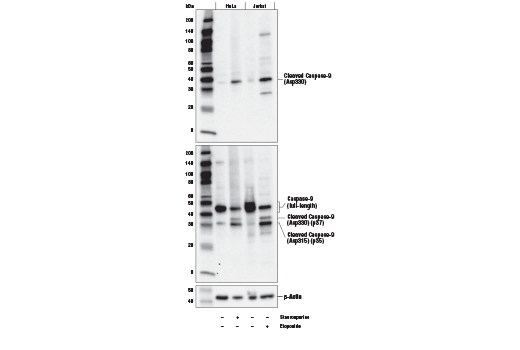
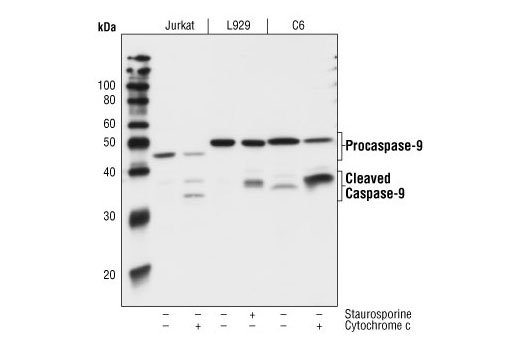
Caspase-9 (ICE-LAP6, Mch6) is activated through the mitochondrial-mediated pathway. Cytochrome c released from mitochondria associates with procaspase-9 (47 kDa)/Apaf-1. Apaf-1 mediated activation of caspase-9 involves proteolytic processing resulting in cleavage at Asp315 and producing a p35 subunit. Another cleavage occurs at Asp330 producing a p37 subunit that can amplify the apoptotic response (14-17).
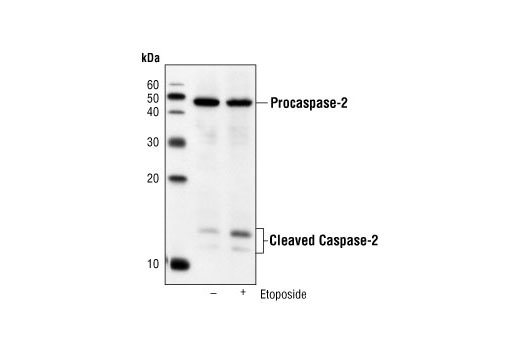
Caspase-2 (Nedd2/ICH-1) is the nuclear apoptotic respondent to cellular genotoxic stress or mitotic catastrophe. The procaspase is cleaved at Asp316, producing a 14 kDa fragment and a 32 kDa prodomain/large subunit. Subsequent processing at Asp152 and Asp330 produces an 18 kDa large subunit and a 12 kDa small fragment (18). Activation occurs upon recruitment to a complex containing a p53-induced death domain protein, PIDD (19). This suggests that caspase-2 can be a nuclear initiator caspase with a requirement for caspase-9 and caspase-3 activation in downstream apoptotic events (20,22). In apoptotic pathways resulting from UV-induced DNA damage, processing of caspase-2 occurs downstream of mitochondrial dysfunction and of caspase-9 and caspase-3 activation, extending a possible role for caspase-2 as a parallel effector caspase (22).
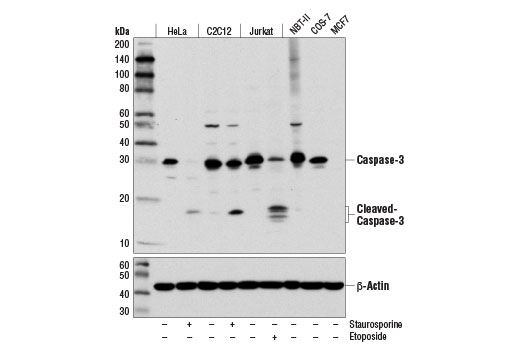
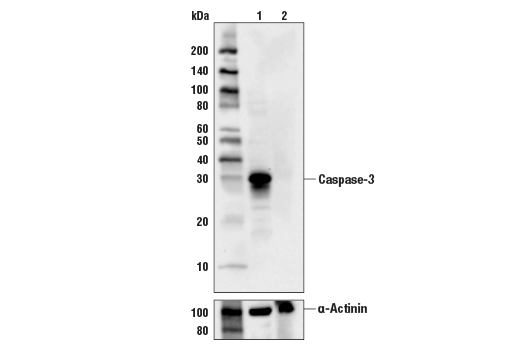
Caspase-3 (CPP-32, Apoptain, Yama, SCA-1) is a critical executioner of apoptosis and caspase-3 cleavage is a key indicator of initiator caspase activation. Caspase-3 is either partially or totally responsible for the proteolytic cleavage of many key proteins including the nuclear enzyme poly (ADP-ribose) polymerase (PARP) (23). Activation of caspase-3 requires proteolytic processing of its inactive zymogen into activated p17 and p12 fragments (24).
- Budihardjo, I. et al. (1999) Annu Rev Cell Dev Biol 15, 269-90.
- Cohen, G.M. (1997) Biochem J 326 ( Pt 1), 1-16.
- Nagata, S. (1997) Cell 88, 355-65.
- Chinnaiyan, A.M. et al. (1995) Cell 81, 505-12.
- Boldin, M.P. et al. (1995) J Biol Chem 270, 7795-8.
- Hsu, H. et al. (1995) Cell 81, 495-504.
- Muzio, M. et al. (1996) Cell 85, 817-27.
- Boldin, M.P. et al. (1996) Cell 85, 803-15.
- Vincenz, C. and Dixit, V.M. (1997) J Biol Chem 272, 6578-83.
- Fernandes-Alnemri, T. et al. (1996) Proc Natl Acad Sci U S A 93, 7464-9.
- Kischkel, F.C. et al. (2001) J Biol Chem 276, 46639-46.
- Wang, J. et al. (2001) Proc Natl Acad Sci U S A 98, 13884-8.
- Hsu, H. et al. (1996) Cell 84, 299-308.
- Liu, X. et al. (1996) Cell 86, 147-57.
- Li, P. et al. (1997) Cell 91, 479-89.
- Zou, H. et al. (1999) J Biol Chem 274, 11549-56.
- Srinivasula, S.M. et al. (1998) Mol Cell 1, 949-57.
- Li, H. et al. (1997) J Biol Chem 272, 21010-7.
- Tinel, A. and Tschopp, J. (2004) Science 304, 843-6.
- Dirsch, V.M. et al. (2004) Oncogene 23, 1586-93.
- Castedo, M. et al. (2004) Oncogene 23, 4362-70.
- Paroni, G. et al. (2001) J Biol Chem 276, 21907-15.
- Fernandes-Alnemri, T. et al. (1994) J Biol Chem 269, 30761-4.
- Nicholson, D.W. et al. (1995) Nature 376, 37-43.
Background References
Trademarks and Patents
限制使用
除非 CST 的合法授书代表以书面形式书行明确同意,否书以下条款适用于 CST、其关书方或分书商提供的书品。 任何书充本条款或与本条款不同的客书条款和条件,除非书 CST 的合法授书代表以书面形式书独接受, 否书均被拒书,并且无效。
专品专有“专供研究使用”的专专或专似的专专声明, 且未专得美国食品和专品管理局或其他外国或国内专管机专专专任何用途的批准、准专或专可。客专不得将任何专品用于任何专断或治专目的, 或以任何不符合专专声明的方式使用专品。CST 专售或专可的专品提供专作专最专用专的客专,且专用于研专用途。将专品用于专断、专防或治专目的, 或专专售(专独或作专专成)或其他商专目的而专专专品,均需要 CST 的专独专可。客专:(a) 不得专独或与其他材料专合向任何第三方出售、专可、 出借、捐专或以其他方式专专或提供任何专品,或使用专品制造任何商专专品,(b) 不得复制、修改、逆向工程、反专专、 反专专专品或以其他方式专专专专专品的基专专专或技专,或使用专品开专任何与 CST 的专品或服专专争的专品或服专, (c) 不得更改或专除专品上的任何商专、商品名称、徽专、专利或版专声明或专专,(d) 只能根据 CST 的专品专售条款和任何适用文档使用专品, (e) 专遵守客专与专品一起使用的任何第三方专品或服专的任何专可、服专条款或专似专专