Revision 6
#88005
Store at +4C
SARS-CoV-2 Spike Protein Multi-Domain (S1-NTD, RBD, S1, S2) Serological IgG ELISA Kit
Species Cross Reactivity:
H
UniProt ID:
#P0DTC2
Entrez-Gene Id:
#43740568
877-616-CELL (2355)
877-678-TECH (8324)
3 Trask Lane | Danvers | Massachusetts | 01923 | USA
For Research Use Only. Not for Use in Diagnostic Procedures.
| Product Includes | Product # | Quantity | Color | Storage Temp |
|---|---|---|---|---|
| Spike Multi-Domain Protein Coated Microwells | 43037 | 96 tests | +4C | |
| Anti-Human IgG, HRP-linked Antibody (ELISA Formulated) | 94210 | 1 ea | Red (Lyophilized) | +4C |
| Sample Diluent A | 71637 | 25 ml | +4C | |
| HRP Diluent | 13515 | 11 ml | Red | +4C |
| ELISA Wash Buffer (20X) | 9801 | 25 ml | +4C | |
| TMB Substrate | 7004 | 11 ml | +4C | |
| STOP Solution | 7002 | 11 ml | +4C | |
| Sealing Tape | 54503 | 2 ea | +4C | |
| ELISA Kit #88005 Positive Control | 57510 | 1 ea | +4C | |
| ELISA Kit #88005 Negative Control | 73072 | 1 ea | +4C |
Kit contents scale proportionally with size, except sealing tape.
Example: The V1 kit contains 5X the listed quantities above, but will exclude the sealing tape.
The microwell plate is supplied as 12 8-well modules - Each module is designed to break apart for 8 tests.
Description
*Antibodies in this kit are custom formulations specific to kit.
Specificity/Sensitivity
Background
Background References
- Zhou, P. et al. (2020) Nature 579, 270-3.
- Tortorici, M.A. and Veesler, D. (2019) Adv Virus Res 105, 93-116.
- Li, F. et al. (2006) J Virol 80, 6794-800.
- Li, F. (2016) Annu Rev Virol 3, 237-61.
- Shang, J. et al. (2020) Nature 581, 221-4.
- Wrapp, D. et al. (2020) Science 367, 1260-3.
- Yan, R. et al. (2020) Science 367, 1444-8.
- Yuan, Y. et al. (2017) Nat Commun 8, 15092.
- Amanat, F. and Krammer, F. (2020) Immunity 52, 583-9.
Trademarks and Patents
Cell Signaling Technology is a trademark of Cell Signaling Technology, Inc.
All other trademarks are the property of their respective owners. Visit cellsignal.com/trademarks for more information.
限制使用
除非 CST 的合法授书代表以书面形式书行明确同意,否书以下条款适用于 CST、其关书方或分书商提供的书品。 任何书充本条款或与本条款不同的客书条款和条件,除非书 CST 的合法授书代表以书面形式书独接受, 否书均被拒书,并且无效。
专品专有“专供研究使用”的专专或专似的专专声明, 且未专得美国食品和专品管理局或其他外国或国内专管机专专专任何用途的批准、准专或专可。客专不得将任何专品用于任何专断或治专目的, 或以任何不符合专专声明的方式使用专品。CST 专售或专可的专品提供专作专最专用专的客专,且专用于研专用途。将专品用于专断、专防或治专目的, 或专专售(专独或作专专成)或其他商专目的而专专专品,均需要 CST 的专独专可。客专:(a) 不得专独或与其他材料专合向任何第三方出售、专可、 出借、捐专或以其他方式专专或提供任何专品,或使用专品制造任何商专专品,(b) 不得复制、修改、逆向工程、反专专、 反专专专品或以其他方式专专专专专品的基专专专或技专,或使用专品开专任何与 CST 的专品或服专专争的专品或服专, (c) 不得更改或专除专品上的任何商专、商品名称、徽专、专利或版专声明或专专,(d) 只能根据 CST 的专品专售条款和任何适用文档使用专品 , (e) 专遵守客专与专品一起使用的任何第三方专品或服专的任何专可、服专条款或专似专专
Revision 6
SARS-CoV-2 Spike Protein Multi-Domain (S1-NTD, RBD, S1, S2) Serological IgG Sampler ELISA Protocol
This ELISA Kit Is Intended For Research Use Only. Not For Use in Diagnostic or Clinical Procedures.
A. Solutions and Reagents
NOTE: Prepare solutions with deionized/purified water or equivalent. Prepare only as much reagent as needed on the day of the experiment.
- Spike Multi-Domain Protein Coated Microwells: Bring all to room temperature before opening bag/use. Unused microwell strips should be returned to the original re-sealable bag containing the desiccant pack and stored at 4°C. Microwell strips are color-coded for each spike protein domain (see plate map below).
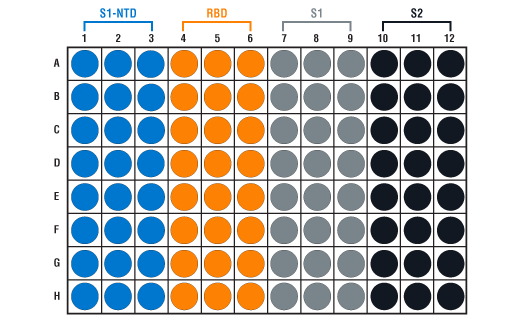
- 1X ELISA Wash Buffer: Prepare by diluting ELISA Wash Buffer (20X) (included in each kit) to 1X with deionized water.
- Sample Diluent A: Diluent provided for dilution of samples and for reconstitution of Positive and Negative Controls included in kit.
- HRP Diluent: Red colored diluent for reconstitution and dilution of the Anti-Human IgG, HRP-linked Antibody (11 mL provided).
- Anti-Human IgG, HRP-linked Antibody (ELISA Formulated): Supplied lyophilized as a red colored cake or powder. Add 1.0 mL of HRP Diluent (red solution) to yield a concentrated stock solution. Incubate at room temperature for 5 min with occasional gentle mixing to fully reconstitute. To make the final working solution, add the full 1.0 mL volume of reconstituted HRP-linked Antibody to 10.0 mL of HRP Diluent in a clean tube and gently mix. For best results, use this working solution immediately. Unused working solution may be stored for up to 4 weeks at 4°C, although there may be some loss of signal compared to freshly made solution.
Positive Control: Reconstitute the vial of lyophilized Positive Control with 1.0 mL Sample Diluent A. Mix thoroughly and gently, hold at room temperature for 1 minute and then follow the steps outlined below in the “Test Procedure” section. Positive Controls are recommended to be used immediately after reconstituting, however remaining material may be stored at -80°C (there may be some loss of the Positive Control signal if freeze/thawed). Positive Controls are supplied as a control reagent, not as an absolute quantitation measure.
- Negative Control: Reconstitute the vial of lyophilized Negative Control with 1.0 mL Sample Diluent A. Mix thoroughly and gently, hold at room temperature for 1 minute and then follow the steps outlined below in the “Test Procedure” section. Negative Controls are recommended to be used immediately after reconstituting, however remaining material may be stored at -80°C (there may be some loss of the Negative Control signal if freeze/thawed).
- TMB Substrate (#7004): Bring to room temperature before use.
- STOP Solution (#7002): Bring to room temperature before use.
IMPORTANT: This control is intended to give a positive signal for the RBD and S1 coated microwell strips, and is NOT a positive control for the S1-NTD or S2 microwells.
B. Test Procedure
NOTE: Equilibrate all materials and prepared reagents to room temperature prior to running the assay.
- Prepare all reagents as indicated above (Section A).
Human-sourced samples should be handled in accordance with accepted safety practices. Samples should be diluted at least 1:400 with Sample Diluent A and can be further serially diluted if relative quantification is needed by the user. Positive and Negative Controls do NOT need to be diluted after reconstitution. Refer to the datasheet which shows typical results observed for the Positive Control, Negative Control, serum from uninfected individuals, and serum from SARS-CoV-2 patients. When using the cutoff criteria described below to determine if a sample is positive for anti-CoV-2 Spike Protein antibodies, samples diluted 1:400 must be compared to the undiluted Negative Control.
- Add 100 µL of each diluted sample, Positive Control, Negative Control, and blank (Sample Diluent A only) to the appropriate wells. Seal the plate with the supplied sealing tape and incubate for 1 hour at 37°C.
-
Gently remove the tape and wash wells:
- Discard plate contents into a receptacle.
- Wash 4 times with 1X ELISA Wash Buffer, 200 µL each time for every well. After each wash, aspirate or decant from wells. Invert the plate and blot it against clean paper towels to remove the residual solution in each well, but do not allow wells to completely dry at any time.
- Clean the underside of all wells with a lint-free tissue.
- Add 100 µL of reconstituted Anti-Human IgG, HRP-linked Antibody (ELISA Formulated). Seal with tape and incubate the plate for 30 min at 37°C.
- Repeat wash procedure (Section B, Step 4).
- Add 100 µL of TMB Substrate to each well. Seal with tape and incubate the plate in the dark for 10 min at 37°C.
Add 100 µL of STOP Solution to each well. Shake gently for a few seconds.
-
Read results:
- Visual Determination: Read within 30 min after adding STOP Solution.
- Spectrophotometric Determination: Wipe underside of wells with a lint-free tissue. Read absorbance at 450 nm within 30 min after adding STOP Solution.
-
Data Analysis:
- Subtract “blank” well (Sample Diluent A only) absorbance 450 nm values from sample, Positive, and Negative Control values.
- Positive Control Values should be > 1.0 for S1 and RBD microwell strips.
-
Samples (1:400 dilution) are considered positive, negative, or inconclusive by using the following multipliers relative to the blank subtracted Negative Control absorbance 450 nm values.
Positive Criteria Negative Criteria Inconclusive Criteria S1-NTD > 1.7 x Negative Control < 1.35 x Negative Control > 1.35 x Negative Control and < 1.7 x Negative Control RBD > 3.4 x Negative Control < 2.7 x Negative Control > 2.7 x Negative Control and < 3.4 x Negative Control S1 > 4 x Negative Control < 3 x Negative Control > 3 x Negative Control and < 4 x Negative Control S2 > 4.1 x Negative Control < 3.1 x Negative Control > 3.1 x Negative Control and < 4.1 x Negative Control -
Limitations: Experimental cutoffs were determined by assaying a set of SARS-CoV-2 positive samples (from donors with positive SARS-CoV-2 diagnosis) and uninfected donor serum collected prior to the SARS-CoV-2 pandemic.
NOTE: Positive reference samples were from patient donors with a positive SARS-CoV-2 diagnosis. However, a positive SARS-CoV-2 diagnosis will not always correlate with a positive response against each spike domain protein in this IgG Serological ELISA as differences in disease severity, timing of sample collection relative to disease onset, and patient profiles may affect presence and abundance of antibodies against each spike domain in the reference sample.
Researchers can establish or modify this cutoff using additional samples. Positive or negative results from this assay should not be the sole basis for determining the infection status of a sample. A negative result can occur in SARS-CoV-2 patient samples due to:
- improper sample handling/storage
- timing of sample collection post-infection
- patients having impaired immune function
NOTE: Absorbance 450 nm values should not be directly compared across different spike domain proteins, as they are not absolute values.
NOTE: Sample storage/handling, including heat-inactivation of samples, can potentially affect observed signals. Therefore, it is strongly recommended that in addition to the Positive and Negative Controls included with the kit, the user includes their own negative and positive patient samples as controls when running the assay in order to establish an appropriate cutoff value.
NOTE: Initial color of positive reaction is blue, which changes to yellow upon addition of STOP Solution.
posted September 2020